SCR (Selective Catalytic Reduction) deNOx System, decarbonization-related catalysts
Kanadevia started developing deNOx catalysts in the 1960s, and in the 1970s, we were the first company in the world to sell commercial-use deNOx catalysts. From the 1970s until today, we have continued to sell deNOx catalysts for more than fifty years, improving them steadily.
In addition, we have been developing catalysts related to decarbonization projects, using the catalyst development technologies we have developed through our SCR systems and deNOx catalysts. While the shipping industry is moving forward with initiatives for decarbonization of marine fuels, as fuels are replaced, issues are arising related to the various alternative fuels, such as the increase of exhaust gas emissions that contain the greenhouse gases of methane and nitrous oxide (N2O), and safety when handling ammonia fuels. Kanadevia is moving ahead with developing catalysts to solve problems such as greenhouse gas emissions reductions, and aims to commercialize them in the near future.
| Alternative fuel | Issue | Solution | Kanadevia-developed catalysts |
|---|---|---|---|
| LNG | Unburned methane (Methane slip) |
Greenhouse gas reductions | Methane oxidation catalyst |
| Ammonia | N2O generation | Greenhouse gas reductions | N2O decomposition catalysts |
| Unburned ammonia (Ammonia slip) |
Utilization of ammonia through deNOx | DeNOx catalysts | |
| Ensuring safety within facilities | Ammonia removal | Ammonia cracking catalyst | |
| Poor combustibility | Co-combustion with hydrogen | Ammonia cracking catalyst | |
| Hydrogen | Transport and storage costs | Hydrogen generation from ammonia | Ammonia cracking catalyst |
| Methanol | NOx generation | DeNOx | DeNOx catalysts by methanol |
Major products
SCR systems and deNOx catalysts
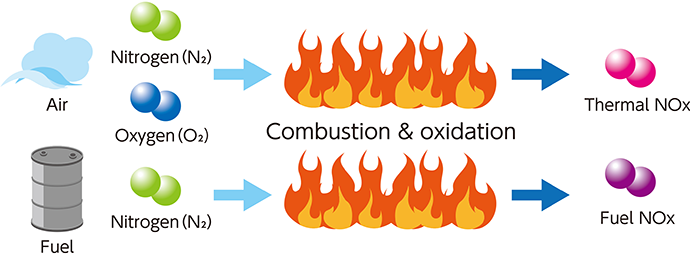
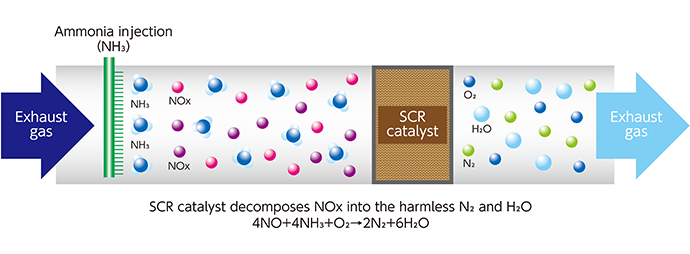
When exposed to the strong ultraviolet rays of the sun, nitrogen oxides (NOx) contained in the exhaust gas emitted from the chimneys of factories and power plants generate photochemical oxidants and PM2.5 particles. These substances are harmful to humans and plants. Furthermore, NOx causes a chemical reaction in the atmosphere to become nitric acid. Nitric acid oxidizes lakes, rivers and soil. It also causes acid rain, which adversely affects plants and organisms. Kanadevia provides denitration catalysts and denitration systems that decompose this NOx by reacting it with ammonia to make it harmless. By doing so, we contribute to the reduction of air pollution.


In addition, NOx is emitted from marine engines, not just land-based plants. Kanadevia began work on our Selective Catalytic Reduction (SCR) system, which draws on our strengths as the only manufacturer in the world of both deNOx catalysts and marine engines, in 2009, and in 2021 we began accepting orders and supplying our SCR system.
(*Our marine engine business was transferred to Hitachi Zosen Marine Engine Co., Ltd., a Group company, on April 1, 2023.)
- Applied technology for SCR systems and deNOx catalysts
Principle
Nitrogen oxides (NOx) are contained in combustion gas emitted from fixed facilities such as thermal power plants, refuse incineration power plants, and chemical plants, and from marine diesel engines. We react NOx with ammonia to decompose them into nitrogen and water, thus making them harmless and reducing NOx emissions.
System components
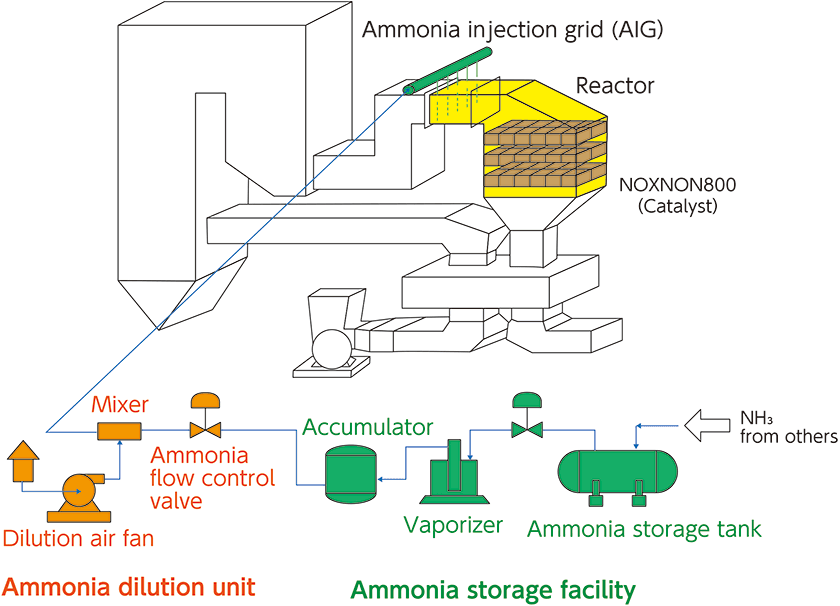
The denitration system consists of a NOx-removal catalyst, denitration reactor, ammonia injection system, ammonia dilution system, and ammonia storage and supply tanks.
DeNOx catalysts

Kanadevia started developing the denitration catalyst in 1969 and commercialized it in 1973. We made various improvements to realize our current product NOXNON 800, which we have supplied since 2016. We supply NOXNON800 not only in Japan but also in the United States, China, and other parts of the world.
Characteristics of catalyst (NOXNON800)
- 1Compatible with a wide range of reaction temperature
- 2Compatible with a wide variety of fuel types
Major records of delivery
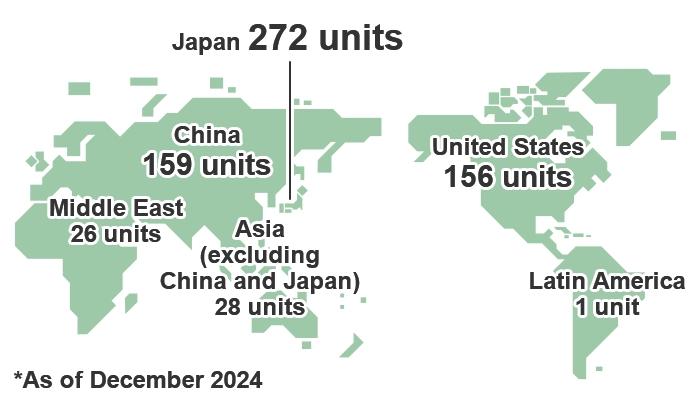
Kanadevia provides deNOx catalysts and deNOx systems to a range of fields, including for gas turbines, boilers (coal-fired, heavy oil-fired, etc.), diesel engines, waste to energy plants , ethylene cracking furnaces, petroleum reformers, and sintering furnaces. Since delivering our first unit in Japan in 1974, we have delivered more than 640 units worldwide, including in the United States and China, Korea, Taiwan, Saudi Arabia, and Qatar. (As of December 2024.) In addition, we have received orders for more than 100 marine SCR systems, which are the applied version of our deNOx system technology. (As of 2021.)
deNOx catalysts by methanol
Background

DeNOx catalysts that use ammonia as a reducing agent can react with SOx (sulfur oxides) in engine exhaust gases to precipitate acid ammonium sulfate inside the reactor, reducing catalyst performance. This is why we developed the deNOx catalyst by methanol that uses methanol instead of ammonia as the reducing agent.
Characteristics of deNOx catalysts by methanol
-
1DeNOx at lower temperatures than urea SCR
Reducing agent Reaction temperature range Peak performance Urea/ammonia 250~400°C Around 350°C Methanol 150~250°C Around 200°C -
2No ammonium sulfate is generated, as there is no ammonia
-
3No evaporator is required, saving space, and direct spraying inside the pipe is possible
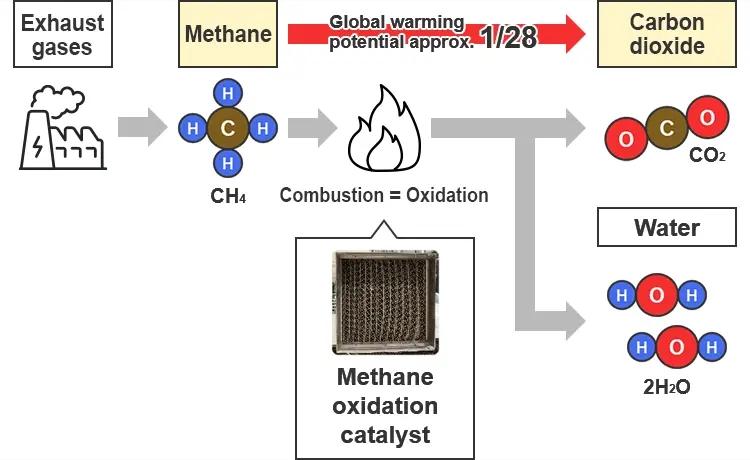
Methane oxidation catalyst
Background
One of the alternative fuels for marine use as we move towards decarbonization is LNG. LNG, or Liquefied Natural Gas, is natural gas, mainly composed of methane, that has been liquefied by cooling. It has fewer greenhouse gas emissions than other fossil fuels, and its price competitiveness is also high compared to hydrogen and biofuel, so it is a key candidate for an alternative fuel. On the other hand, there are concerns about it generating methane slip to the atmosphere as the unburned methane is emitted alongside the exhaust gases. As methane has a global warming potential about 28 times greater than carbon dioxide, methane oxidation catalysts with high methane slip reduction rates are in demand in order to increase the greenhouse gas reduction effects of LNG.
Principle
Unburned fuel methane contained in engine exhaust gases is oxidized using a catalyst and emitted as carbon dioxide, which has a smaller global warming potential than methane, thereby helping reduce the environmental impact.
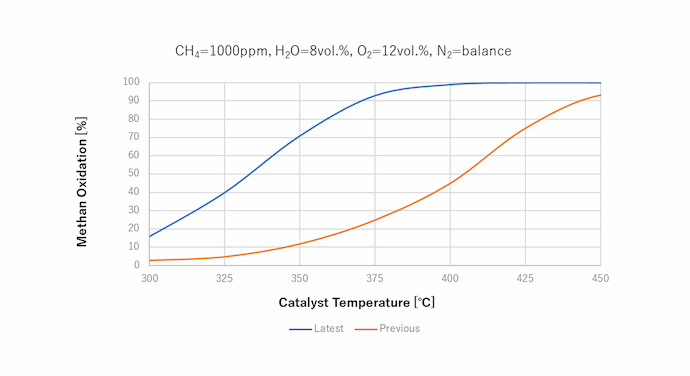
Characteristics of our methane oxidation catalyst
-
1Achieves a 70% methane oxidation rate at a lower temperature (350°C) than conventional methods
Major records of delivery
Kanadevia's methane oxidation catalyst is being used in a project to develop technology to reduce methane slip from LNG-fueled ships through catalyst and engine improvements (hereinafter, "this Project"). This Project is being carried out as part of the Green Innovation Fund's Next-generation Ship Development project , run by the New Energy and Industrial Technology Development Organization (hereinafter, "NEDO") which started in FY2021.
In land-based testing for this Project, a reduction rate of 93.8% (with engine load at 100%) was achieved, and the catalyst was the first in the world to receive a certificate from ClassNK verifying that we had achieved this reduction rate. Starting in FY2024, the verification test at sea has started using a large coal carrier vessel.
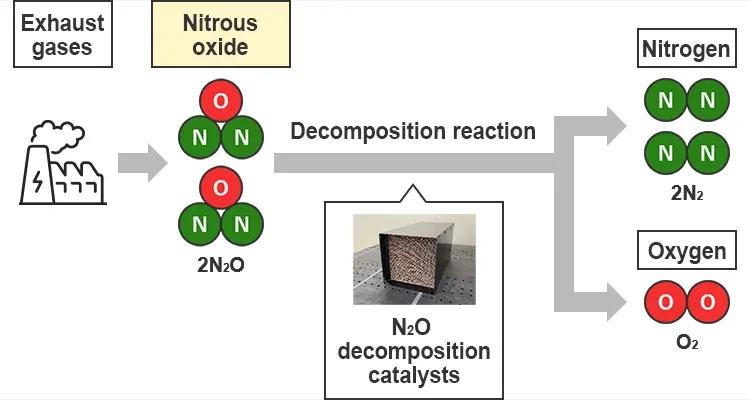
N2O decomposition catalysts
Background
Because ammonia does not emit carbon dioxide when burned, it is expected to be an alternative to fossil fuels as we move towards decarbonization, and in Japan as well, progress is being made on the development of ammonia engines for ships. At the same time, the global warming potential of the N2O generated when ammonia is oxidized is huge, about 265 times that of carbon dioxide, so there are concerns that if this is just released as is, it will worsen the greenhouse gas reduction effects. To improve the greenhouse gas reduction effects, it is important to use a catalyst to decompose N2O into nitrogen and oxygen, so there is a need for N2O decomposition catalysts that enable more efficient decomposition.
Principle
While ammonia engines emit N2O, which has a global warming potential about 265 times that of carbon dioxide, efficiently decomposing the emitted N2O using a catalyst contributes to reducing the environmental impact.
Characteristics of our N2O decomposition catalysts
-
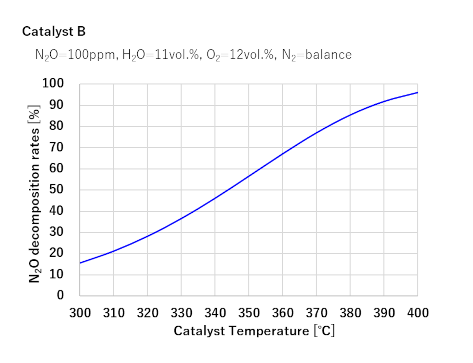
1Two types of catalysts under development achieve nearly 100% N2O decomposition rates at 400°C
(Marine engine exhaust gases are no more than 400°C)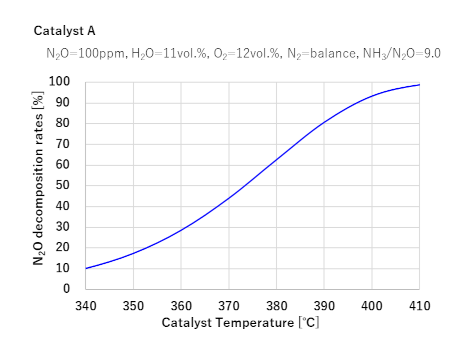
-
2We are developing a comprehensive ammonia exhaust gas processing system through a combination with other Kanadevia catalysts.
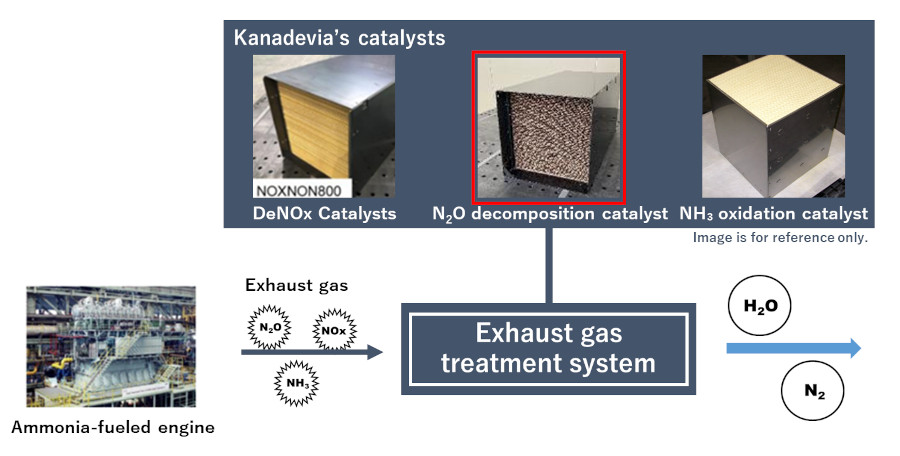
Major records of delivery
Kanadevia's N2O decomposition catalyst has also been selected for the "Development of N2O reactor installed on ammonia-fueled ships" project under NEDO's Green Innovation Fund Next-generation Ship Development project from FY2024. Verification testing using actual ships is scheduled to start in FY2026, and we are working on development with the goal of widespread use of N2O decomposition catalysts.
Ammonia cracking catalyst
Background
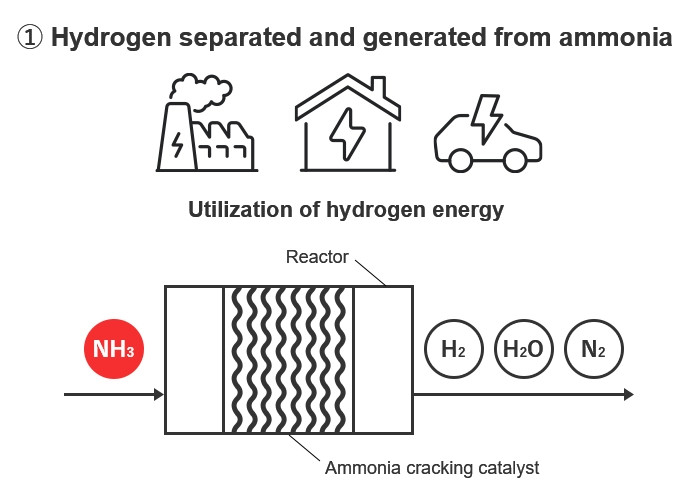
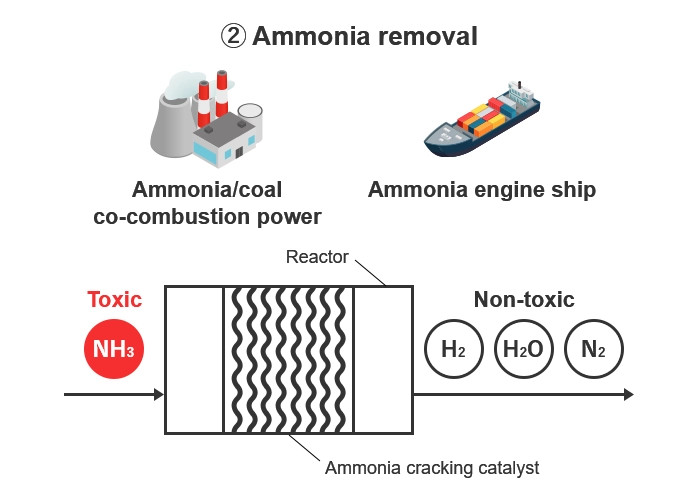
The technology of heating ammonia to break it down into hydrogen and nitrogen is called ammonia cracking (decomposition), so a catalyst used in ammonia cracking technology is called an ammonia cracking catalyst. Its main uses are (1) separating and generating hydrogen from ammonia , and (2) removing ammonia . The hydrogen separated and generated from ammonia is used in fuel cells, thus solving the problem of hydrogen transport and storage. Also, it helps improve safety for facilities by eliminating ammonia safely from pipes in facilities that use ammonia such as ammonia engines.
Principle
Normal ammonia cracking catalysts supply the heat needed for decomposition externally through burners or heaters, etc. However, Kanadevia's catalysts use an internal heat supply system that ensures the required heat through the oxidation of part of the ammonia.
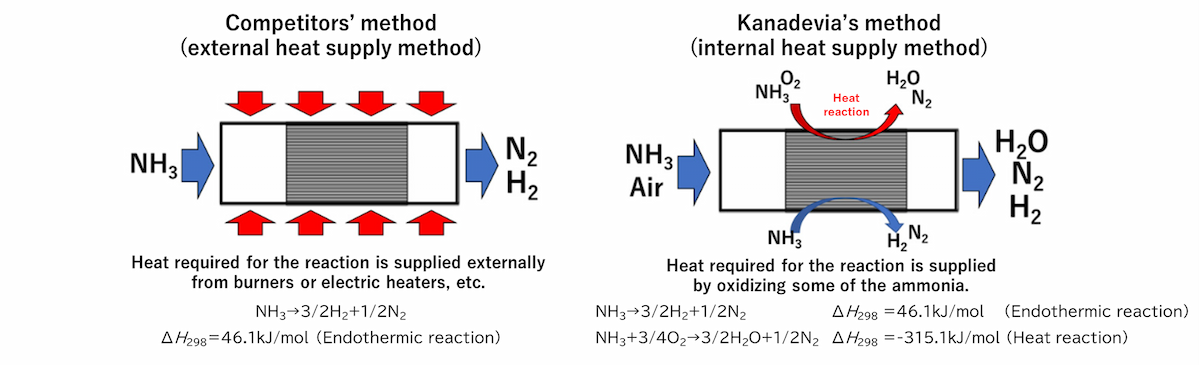
Characteristics of our ammonia cracking catalyst
-
1Cheaper than normal ones that use precious metals as it uses base metals
-
2While catalyst shapes are normally granular, ours have a honeycomb shape, which reduces pressure loss
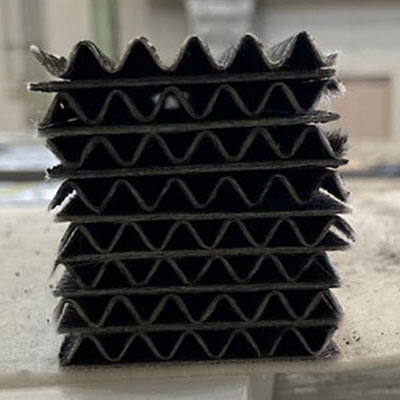
-
3As heaters, etc. are only used to supply heat at the initial start-up, the heating time is reduced compared to the external heat supply method
(Heating is not required during normal use)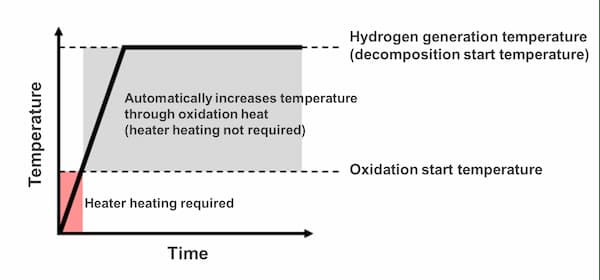
Related technology
Click here for inquiries about Kanadevia technology


